Latest Features
Pulmonary Oxygen Toxicity: Expanding Our Understanding With Two New Models
Last September, InDepth presented new data-driven models to predict the risk of pulmonary oxygen toxicity on a dive, which arguably represent an advancement over the legacy REPEX method (OTUs). Now our resident dive geek Reilly Fogarty applies these models to predict and compare the risk results on two BIG tech dives: Italian explorer Massimo Bondone’s eight-hour jump to 199 m/650 ft on the SS Brandenberg near Tuscany, Italy, and Dr. Andy Pitkin’s 124m/404 ft deep, 12-hour runtime push dive at Weeki Wachee Springs, Florida. The results will get your lungs pumping!
By Reilly Fogarty
See Previous InDepth Story: How To Calculate the Risk Of Pulmonary Oxygen Toxicity by Ran Arieli
These days you would have to be a lunatic (J-valve fanatics, I’m looking at you) to dive without a pressure gauge. Without a gauge, you can’t know how much gas you have left: whether you’re riding the edge of your reserve supply or have enough gas to finish your dive and then some. Planning a dive without understanding oxygen toxicity risk calculations is just as dangerous, but a little more complicated to work through.
Unfortunately for new divers, there just aren’t enough synonyms for “glossing over” to illustrate the number of ways we avoid delving into the complicated science of pulmonary oxygen toxicity management via misdirection, well-meaning anecdotes, or junk science. Like many research theories, what we know about the physiology of the condition is still evolving. Simplified perspectives based on antiquated calculations have a way of initiating a cyclical pattern of compounding mistakes, as one diver explains their best understanding to another, with each diver adding some element of inaccuracy. Despite the challenges, it’s our job as divers to understand how and why we make the decisions that affect our safety.
No single article can hope to cover the scope of a topic like this, but our aim is to provide you with the necessary tools to begin to understand the physiology of pulmonary oxygen toxicity. Our modern understanding is closely intertwined with the history of research into the subject, so while our hope is to educate, we also want to avoid causing harm through misinformation or to give the false impression of complete understanding. Risk mitigation falls to you, as does comprehending the material before you attempt to apply it in your own diving.
The broad strokes of this research can be best understood through the evolution of the unit pulmonary toxicity dose (UPTD) model, the derived equation for repetitive exposure (REPEX) model, and modern approaches developed by Barbara Shykoff, PhD, and Ran Arieli, PhD. Much like the decompression models we use, these are largely (in some cases wholly) unproven models based on compounding theories. Interestingly, the oldest and most problematic of these methods remains the most-used and least frequently questioned by divers. Here’s what you need to know about pulmonary oxygen toxicity.
The Unit Pulmonary Toxic Dose (UPTD) Model
The UPTD model was first developed in 1970 by researchers at the University of Pennsylvania. The theory is based on the loss of vital capacity (VC – the maximum volume of gas that can be voluntarily moved into or out of the lungs) that the researchers believed correlated with the symptoms of pulmonary oxygen toxicity.
The researchers used a small sample group of young men to create a dataset on oxygen exposure symptoms. These participants were subjected to a range of oxygen exposures with PO2 ranging from 1.0 to 3.0 ATA over a variety of exposure durations from 3.5 to 24 hours. To compare exposures across PO2 and exposure time differences, a common unit was created—the UPTD. This unit was defined as the equivalent to one minute of exposure to 100% oxygen at one bar. UPTD could then be calculated as follows and exposures could be compared:
By converting exposures to a common unit, the researchers believed that dives could be compared across depth and gas choices, and repetitive dives could be compared by the sum of their exposure values.
Shortly after the initial work was published, the cumulative pulmonary toxic dose (CPTD) model was created to allow for continuous oxygen exposures above a PO2 of 0.5 ATA to be calculated and expressed in UPTD. As a result of this work, a rudimentary understanding of the onset and presentation of pulmonary oxygen toxicity symptoms began to evolve. It’s important to note, however, that the original UPTD model did not differentiate between dives performed one hour or 23 hours apart—there was no calculation for complete or partial recovery.
UPTD is now often shortened to oxygen toxicity units (OTUs) and the calculations are still widely used even 45 years after their development. It remains unclear whether the model is accurate or the results of UPTD calculations merely overlap adequately with safe limits under common diving conditions, but experts are increasingly finding fault with the model.
Notably, Barbara Shykoff, PhD and US Navy Experimental Diving Unit (NEDU) researcher, has cited several past works from Duke University, Clark, Lambertsen, and others in critique of UPTD applications in the real world. Among her complaints are several significant deviations from the model’s predictions among dives performed at PO2 other than 2 ATA, differences in underlying lung injury and physiology, and disparity between VC changes at mild and extreme PO2 exposures. Research from Clark and Lambertsen has also indicated that the “unit dose” concept may not be applicable in a linear fashion above a PO2 of 1.5 ATA, but requires calculation as a “function of time squared for higher PO2s”.
The Derived Equation for Repetitive Exposure (REPEX) Model
If UPTD was a best-fit model designed for a small data set, the NOAA REPEX model took that applied theory a step further. Developed by R.W. “Bill” Hamilton, PhD, in the early 1980s, the NOAA-derived equation for repetitive exposure (REPEX) model was designed to facilitate recovery and multi-day diving calculations. The model was notably never validated in divers but was built on the concepts of the original UPTD and CPTD model and other supporting studies.
Hamilton’s team set theoretical limits for various exposures, with 850 OTU being an allowable single day exposure but gradually reducing the average daily OTU as missions became longer. He did note that these exposure limit curves would have to be modified with operational experience or desired conservatism, but did not verify the curves with real-world trials. The most often-voiced concern about this model is that it is based on a linear extrapolation of prior data, data that may itself not be accurate. This leaves it with a number of significant flaws and potentially erroneous results, particularly outside the relatively narrow range of exposures for which the UPTD system was designed.
Supporters of the REPEX system might dispute those assertions. It is fair to point out that the NOAA oxygen exposure limit tables have been used extensively for several decades with a great deal of success. This success could be due to accuracy of the model within the applied parameters common in recreational diving. It could also be the result of significant conservatism caused by a large gap between the calculated limits and the real-world exposure limits that incur pulmonary toxicity risk.
Shykoff’s Calculator for Estimating the Risk of Pulmonary Oxygen Toxicity
Shykoff’s work is one of the first notable evolutions in the way we proposed methods to calculate pulmonary oxygen toxicity risk in decades. In a paper published in 2015, she outlines a method by which divers can calculate risk over the course of repeated dives. The method is centered around what Shykoff calls a “incidence-time model” that stipulates a linear relationship in a mid-range exposure value. This makes it possible to not only calculate oxygen toxicity for duration, but also extrapolate equivalent exposure time between exposures at different PO2s. This is coupled with a recovery model and centered around the idea that any dive with a PO2 of 1.3 to 1.4 bar begins some level of pulmonary injury. Shykoff admits that the probability of noticeable injury in small exposures is quite small, but she believes there is a proportional risk between this and intermediate duration exposures. This differs from the UPTD and REPEX models in that it significantly limits its extrapolation of linear relationships only to the intermediate exposures, notable because exposures beyond a small midrange have indicated significant variance from the norm in prior models.
Also important is the verification of Shykoff’s model. While its design relies on a relatively small dataset of 1350 in-water dives, the fact that it has been verified in divers at all separates it from its predecessors. These dives occurred with a PO2 of between 1.3 and 1.4 bar, and were used to create and verify the time-incidence model. An additional dataset using 620 dives was used to validate the recovery model used in Shykoff’s risk calculator.
After resting dives: ▲ / After dives with exercise: ■
The lines are the best fit models fitted to the data:
–––– for resting dives / – – – for dives with exercise,
tr/Tdur = exp[–k (t/Tdur)2], k = 0.149, 0.047 for resting dives or those with exercise, respectively.
Graphic courtesy of Babara Shykoff.
Interestingly, these data showed that significant recovery did not occur until nearly five hours after a dive, with no measurable recovery apparent after two separate three-hour dives that occurred with a two-hour surface interval between them. Whether this is a function of actual recovery mechanisms or a limitation of the model remains unclear.
In terms of practical application, Shykoff’s model makes a few interesting points. Most notably, she suggests completing shorter dives before longer ones, and a resting dive before one with exercise. This is both to avoid a possibly over-conservative calculation of risk due to the rate of recovery being calculated with the duration of exposure in the denominator, and because recovery after a dive that involves exercise occurs at nearly half the rate as one done at rest. It’s also important to note that zero incidence does not correlate with zero duration. Shykoff cites a 95% confidence limit for this model at 0.8 – 4.9%, meaning that—purely by the numbers—approximately 3% of divers in the model may exhibit signs or symptoms of pulmonary oxygen toxicity at the beginning of the dive with no exposure to elevated PO2. This point must be followed with the note that this is directly comparable to the overall incidence of symptoms found in a dataset of 239 shallow open-water dives with a PO2 of 0.3 bar (resulting in about 6% symptom incidence) and can be “interpreted as the effect of breathing underwater.”
Another key differentiation from prior models is the recovery rate calculation. While prior models used a first-order kinetic model, Shykoff used a sigmoidal shape recovery pattern based on real-world data. This means that recovery rates vary between their initial rate, a maximum rate, and a final slower rate in a non-linear fashion. More complex calculations alone don’t imply superiority, but Shykoff does have some real-word data to back up her model. Experimentally, it appears that injuries from one exposure may begin to diminish even as damage from a following dive occurs, leading to occasional improvement of symptoms in subjects who began a dive with symptoms (note that this is an experimental observation and NOT a recommendation or suggestion of causation).
This model also does not associate severity of oxygen toxicity with exposure duration. It also does not differentiate between severity of exercise, other effects of mild hyperoxia, or PO2 significantly deviating from 1.3 bar or beyond a single exposure of up to eight hours. The model is based on a small dataset, and its results should be treated as almost entirely theoretical in their current form.
Arieli’s Toxicity Index Derived from the Power Equation
Ran Arieli, PhD is the former head of Hyperbaric Physiology Research at the Israel Naval Medical Institute, and takes many of the same issues with the UPTD and REPEX models that Shykoff and their colleagues do. In response, he’s developed a method that focuses on a power-law approach to pulmonary oxygen toxicity risk calculations. This model focuses on the polynomial expression of reactive oxygen and nitrogen species that can be correlated with pulmonary injury, and the assumption that the development of these species is related to the highest power of the PO2 exposure. Combining this with other oxygen toxicity symptoms (decrease in lung capacity, ventilatory drive, or thickness of alveolar wall, for example) modeled by their exposure time, the rate of hydrogen peroxide (a reactive oxygen and nitrogen species precursor) can correlate with the square of time and—Arieli believes—can provide an ability to predict oxygen toxicity risk.
More on how Arieli derived his power equation can be found in a previous InDepth article, “How To Calculate the Risk Of Pulmonary Oxygen Toxicity,” but at its core his model was designed to address a demonstrable difference in pulmonary pathologies found at high and low PO2 exposures. Modeling different risk factors for each resulted in a model that Arieli believes more accurately represents pulmonary oxygen toxicity risk.
This model has the added benefit of being directly verifiable through the measurement of reactive oxygen and nitrogen species, or their precursors. The caveat to this is a correlation between these species, and the expected pulmonary pathology must be assumed, although these relationships are relatively well accepted. Like Shykoff’s model, Arieli’s proposed model has fairly specific parameters. Its initial publication specifically focuses on interpolating risk for exposures done under sedentary to light intensity activity (1 to 4.4 MET) in subjects either dry or immersed in 33°C water. The result is a model which Arieli believes can accurately be used to estimate pulmonary oxygen toxicity risk in divers but which must be carefully applied within the parameters of its original intent.
Applying The Models to Real World Dives
The SS Brandenberg

It’s hard to bring concepts like these into focus without crunching some numbers, so working through an example of a long CCR dive can help illustrate the differences. Take a dive like Massimo Bondone’s exploration of the SS Brandenburg for example. A British steamship torpedoed on February 10th, 1941, the Brandenburg now sits in nearly 199 m/650 ft off the coast of Tuscany, Italy. The Italian explorer and his team identified and dived the wreck in 2017. Their efforts represent the fourth deepest shipwreck exploration dive conducted by tech divers.
Using a closed-circuit rebreather Bondone logged 15 minutes of bottom time and a 490 minute total run time for his dive. This dive is extreme, and difficult to do without significantly exceeding CNS toxicity limits under almost all guidelines, but useful for exploring the limits of pulmonary toxicity which take greater total exposure time to exceed. Assuming a constant PO2 of 1.0 bar, here’s how each of the models works out:
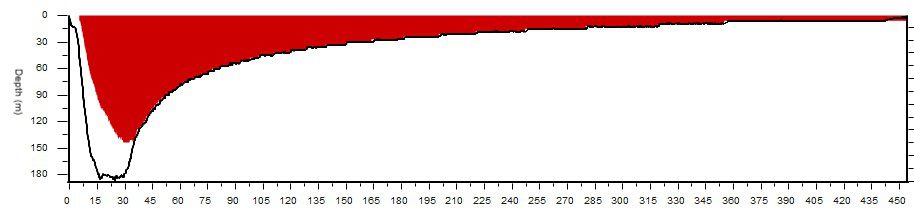
UPTD:
Total toxicity in UPTD can be calculated as follows:
UPTD = t * [(PO2 – 0.5)/(1-0.5)]1/1.2
UPTD = (490 minutes) * [1.0 – 0.5)/(1-0.5)]1/1.2
UPTD = 490
This exceeds the latest US Navy Diving Manual limit for a daily exposure (450 UPTD), although a single extreme exposure limit of 1425 UPTD is designated for medical applications in the operational theater. Bondone’s dive was extreme, but UPTD guidelines put it just beyond the allowable limits for single-day pulmonary oxygen toxicity exposure.
REPEX:
That same dive by REPEX guidelines results in the same unit value – 490 OTU – but has significantly shorter maximum exposure durations.
By these guidelines, a 490 minute exposure to a PO2 of 1.0 bar is more than two hours over the limit for single exposures. There is no way to quantify the increase in risk once the limit is exceeded; the guidelines were designed to be binary. It is interesting to note how much shorter than the UPTD guidelines the REPEX exposure limits are.
Shykoff’s Method:
The Shykoff method is significantly more involved to calculate by hand, but is available to easily calculate via an online tool Shykoff has published. One issue with calculating this example dive, however, is that the model has been designed only for a PO2 range of 1.3 to 1.4 bar – the lower PO2 ranges commonly used for dives like this fall outside the intended range of the model. Calculating the dive with a higher PO2 but an identical dive time (something that might occur with more conservative gradient factors, for example) gives the following:
Unlike the older models, this calculation gives a probability of symptoms, rather than just a binary judgement on whether the dive fits within acceptable parameters or not. That probability does not imply accuracy in and of itself, but if the mode proves accurate it could provide new possibilities for dive planning in extreme exposures.
Arieli’s Method
Calculating toxicity risk by the power equation derived index method that Arieli has published requires a bit of work. For PO2 greater than 0.6 bar, the following equation results in the POT index:
POT Index = T2* PO24.57
This yields a POT Index of 49. This value can then be used to estimate incidence of toxicity symptoms via the following:
Incidence (%) = 1.85 + 1.071 * POT Index
This value, as estimated by the model and the dataset from 16 HBO exposures performed at the NEDU, results in an estimated 54% likelihood of pulmonary toxicity symptom evolution. Within the limitations of Arieli’s dataset, the predictions appear to have a strong correlation to real-world results, and the calculated risk is similar to that found using Shykoff’s model. Bondone reported that though he has had pulmonary oxygen toxicity symptoms on dives, he did not experience any symptoms after diving the Brandenberg.
Weeki Wachee

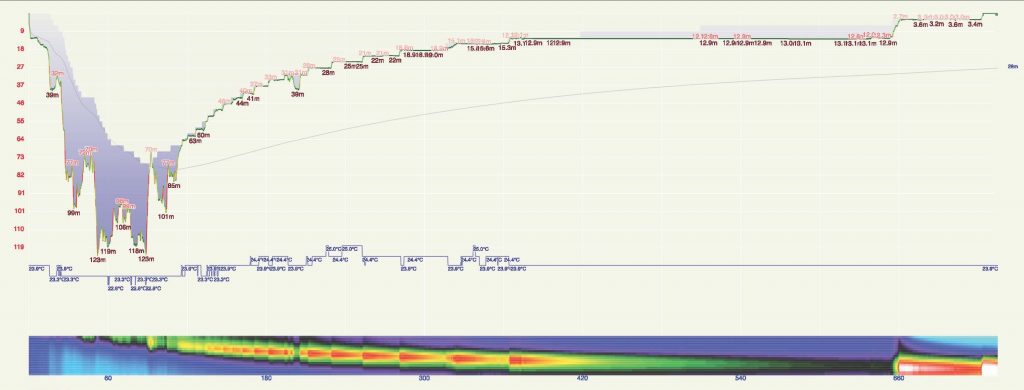
Pulmonary oxygen toxicity comes to the forefront of diver’s concerns as their expeditions grow longer, so there’s value in looking at a longer, slightly shallower dive as well. Dr. Andy Pitkin is a pediatric cardiac anesthesiologist and researcher who regularly participates in dives exploring some of the deepest caves in the United States. In 2019, he and his teammates performed a dive to a maximum depth of 124 m/404 ft in just over 12 hours while exploring the Weeki Wachee Springs system with the Karst Underwater Research (KUR) group.
The dive involved years of work-up and an innovative array of support personnel, equipment, underwater habitats, and emergency planning, but it’s the effect of the extreme exposure to elevated PO2 that we’re interested in here. Using a rebreather with a setpoint of 1.1 bar, Pitkin spent 725 minutes underwater. Ignoring the air breaks used to manage CNS toxicity near the end of his profile, here’s how each model breaks it down:
UPTD:
Here’s how Pitkin’s dive looks calculated via the original UPTD algorithm:
UPTD = t * [(PO2 – 0.5)/(1-0.5)]1/1.2
UPTD = (725 minutes) * [1.1 – 0.5)/(1-0.5)]1/1.2
UPTD = 843
Like Bondone’s dive, this exceeds the US Navy Diving Manual limit for daily exposure, this time by almost double (450 UPTD vs 843 UPTD). However, the allowable limit for a single extreme exposure of 1425 renders this technically within some limits, although those were intended only for medical applications in emergency situations. By UPTD standards, this dive would be significantly beyond any reasonable single-dive exposure.
REPEX:
Just as with Bondone’s dive, the REPEX calculations provide the same 843 OTU value as the UPTD calculations. In this case, the exposure is even further past the NOAA Diving Manual guidelines for a maximum single exposure (just 240 minutes at the working PO2 of 1.1 bar). It would not be possible to perform this dive via either REPEX or UPTD guidelines.
Shykoff’s Method:
It’s impossible to calculate this dive within the confines of Shykoff’s method because she designed her model around a working PO2 of 1.3 to 1.4 bar. Attempting to calculate a dive this long at an artificially high PO2 results in dramatic numbers, but none that reflect reality. Because Pitkin chose to run a lower PO2 to attenuate the risk of CNS oxygen toxicity, this dive falls below the more common working PO2 used by rebreather divers around which Shykoff has designed her model.
Arieli’s Method
This dive falls significantly beyond the exposure guidelines that Arieli has set forth, but his are less binary than those used by Shykoff’s model. Calculating POT Index as follows:
POT Index = T2* PO24.57
And theoretical incidence via:
Incidence (%) = 1.85 + 1.071 * POT Index
Results in a value of 240%, or 240% likelihood of pulmonary toxicity symptom evolution.
It’s results such as these that illustrate the fact that these models are entirely theoretical to those who are first playing with the data. Particularly as exposures deviate from the norm, their results will appear increasingly extraordinary, but none of those results may reflect reality in the first place.
Pitkin completed his dives with minimal symptoms, despite all four of these models indicating that it could be extremely hazardous to use such a profile. Pitkin explained that he and his partners frequently experience some low-grade symptoms of pulmonary oxygen toxicity following similar dives. These symptoms typically include “substernal tightness, mild cough, and the feeling of inability to take a deep breath,” which is why they now limit the PO2s on these dives. Symptom onset has become an expectation on dives such as these but severity is decreased by using a setpoint of 1.2 bar or less (interestingly, according to Pitkin, time to symptom onset is not improved by this change). “A bonus is you don’t feel as crappy afterwards,” he said.
Each of these four models has indicated the likely onset of pulmonary toxicity symptoms from this profile, and while symptoms have occurred they are perhaps milder than some models would suggest. This somewhat mixed result is indicative of the confusing nature of this type of modeling – Pitkin noted that, “there is a lot of inter-individual variation in susceptibility to pulmonary oxygen toxicity,” and this variability likely presents enormous challenges to accurate risk modeling.
We conferred with Arieli regarding these results. He noted that some recent research indicates the possibility of acclimatization to high PO2 exposures in technical and CCR divers that could explain lower than expected rates of CNS oxygen toxicity. This could potentially apply to pulmonary toxicity events as well.
Takeaways
The difficulty with covering a topic like this is the desire to educate but not instill undue confidence in divers. Knowledge is a powerful thing, and it’s easy to see research that agrees with what we’ve been taught and immediately apply it to our own diving. My hope is that you can take this article, use it to fill in the gaps in your own knowledge, and find the areas where you’d like to dig a little deeper.
UPTD and REPEX models may be inaccurate (wildly so in some applications), but many divers have been trained to use them in ways that promote safe diving practices and result in low injury incidence. Similarly, Shykoff and Arieli’s methods are exciting and appear accurate but lack the real-world testing to verify their efficacy. They are significantly hindered by their parameters, and we have yet to determine if they represent a more accurate model at large, or just a closer correlation to a limited dataset.
Like many elements of diving research, the potential here is enormous. While you may be left waiting for more research before you can change the way you plan your dives, it’s likely that these two models and those that will come from them will dictate how we dive in the future. Knowing how we got to this point, what the pitfalls of our current calculations are, and how researchers hope to remedy those pitfalls in the future is valuable and can contribute to your safety. Dig into the details wherever possible, see how each model calculates your most challenging dives, and think about how you can make your dives safer with all of the knowledge available to you.
A Special Thanks to Massimo Bondone and Dr. Andy Pitkin for sharing their dive data and Neal Pollock, Ph.D. for lending his expertise.
Dive Deeper:
InDepth: How To Calculate the Risk Of Pulmonary Oxygen Toxicity by Ran Arieli
Frontiers in Physiology: Aviner B., Arieli R.,Yalov A. Power equation for predicting the risk of central nervous system oxygen toxicity at rest
Arieli R., Aviner B. Acclimatization and deacclimatization to oxygen: determining exposure limits to avoid cns o2 toxicity in active diving
Calculator For Estimating The Risk Of Pulmonary Oxygen Toxicity by Dr. Barbara Shykoff
Shearwater Research: Why UPTD Calculations Should Not Be Used by Barbara Shykoff, 2017
Spums Journal: Tolerating Oxygen Exposure by RW Bill Hamilton, 1997
RW Bill Hamilton’s Original REPEX paper: Tolerating Exposure To High Oxygen Levels: Repex And Other Methods by RW Hamilton, 1989An early 1985 review of the UPTD Model: Predicting Pulmonary O2 Toxicity: A New Look at the Unit Pulmonary Toxicity Dose by AL Harabin, L.D. Homer, PK Weathersby and ET Flynn

Reilly Fogarty is an expert in diving safety, hyperbaric research, and risk management. Recent work has included research at the Duke Center for Hyperbaric Medicine and Environmental Physiology, risk management program creation at Divers Alert Network, and emergency simulation training for Harvard Medical School. A USCG licensed captain, he can most often be found running technical charters and teaching rebreather diving in Gloucester, Massachusetts.